Important Announcement
Parents, please do NOT send your child to school if he or she has a pending COVID-19 test. Thanks for your cooperation in this matter.
News and Social Media

Upcoming Events

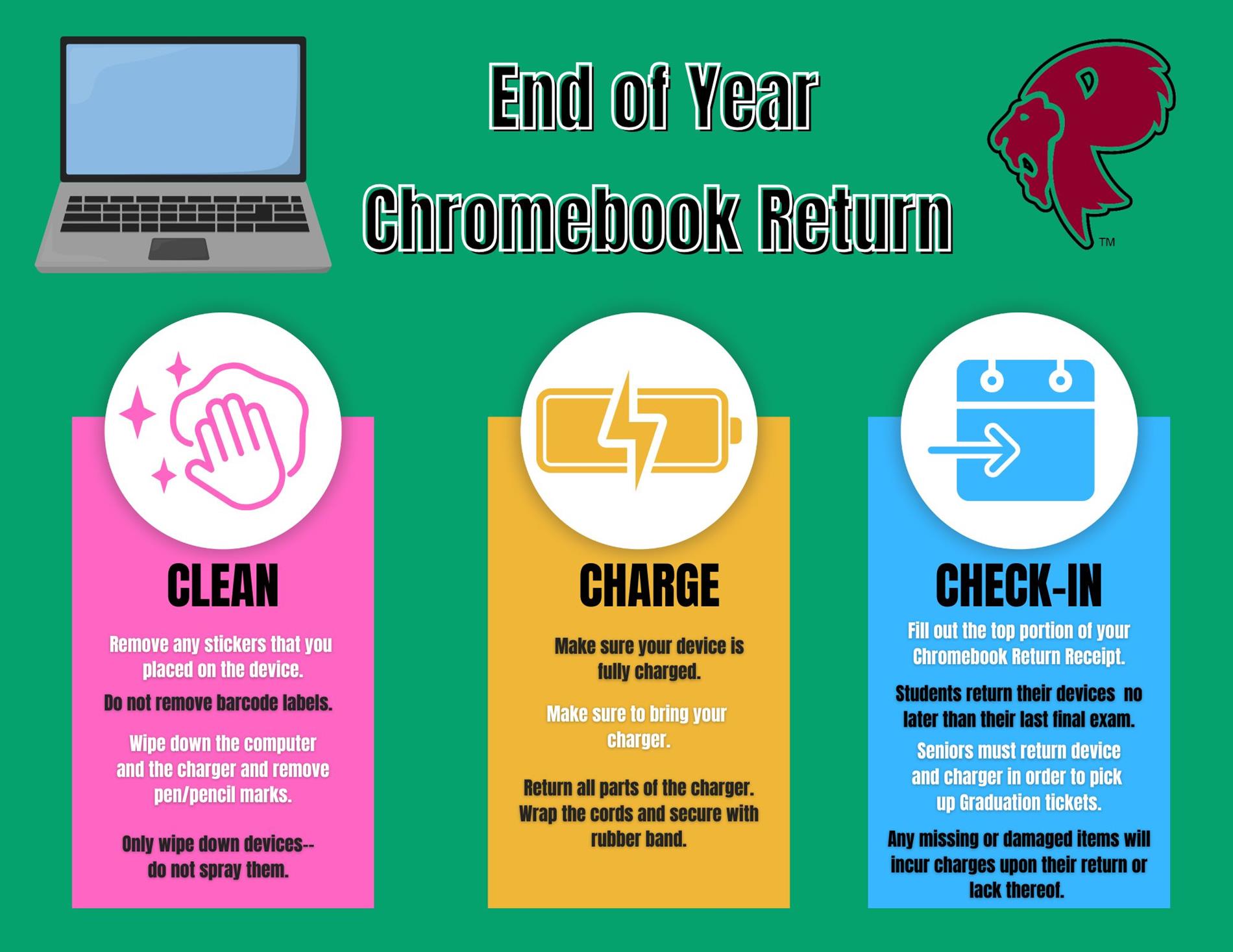
2025 Chromebook Return Instructional Video

FAFSA Information
Student Email Account Login
Students, use this format when accessing school email accounts:
last name.last 4 of student # @student.acboe.net
PHS Alabama Continuous Improvement Plan 2023-2024
Parents and students, please review the Cell Phone Procedures.
Stanley-Jensen Stadium Policies





















